Getting a better look at living cells
Nanoscale-level imaging of living cells has become a reality in the past few years using transmission electron microscopy and sealed sample holders that keep cells alive in a liquid environment. But do the high-resolution images obtained using these tools truly reflect the structures and functions of cells, or do they show cells damaged by the high-intensity electron beam used in transmission electron microscopy?

Tolou Shokuhfar, associate professor of bioengineering at the University of Illinois at Chicago College of Engineering.
“We really have had no way of knowing if what we see in images obtained through liquid cell transmission electron microscopy show the natural state of cells, or if the morphological changes we see are actually the result of radiation damage,” said Tolou Shokuhfar, associate professor of bioengineering at the University of Illinois at Chicago College of Engineering.
Shokuhfar and colleagues describe a device that works with most transmission electron microscopes that would significantly reduce the exposure of live samples to the electron beam used in transmission electron microscopy. They report their results in the journal Science Advances.
Transmission electron microscopy produces incredibly detailed images of cells that can show structures as small as one or two nanometers across. But for a long time, samples used in transmission electron microscopy had to be dead or frozen because the sample chamber of a transmission electron microscope is a vacuum.
The new field of liquid cell transmission electron microscopy emerged in recent years enabling scientists to study biological, chemical and materials science samples in their near-native environments. This is achieved by placing the sample in liquid inside a tiny sealed chamber that protects it from the high vacuum environment to allow dynamic imaging.
However, currently-available devices that hold samples only allow for a single chamber to be placed under the microscope at a time. “Because you place just one sample at a time under the microscope, you need to perform your pre-imaging focus and setting adjustment on that one sample,” said Trevor Moser, a graduate student at Pacific Northwest National Laboratory in Richland, Washington and a co-author on the paper. “By the time you are ready to take pictures, the sample has already been exposed to significant amounts of radiation, so you just never know if the pictures you get show the unaltered cell, or if what you see on the pictures is because of damage from the electron beam,” continued Moser, who has previously worked in Shokuhfar’s lab.
The research team solved this problem by developing a device with 25 transparent windows rather than the single window sample holders currently provide. With more windows, the researchers expose samples to less radiation by getting closer to the settings and focus they need using one of the windows and then switching to another window where cells haven’t yet been exposed to the radiation from the microscope’s electron beam. Researchers still need to focus on samples in the ‘fresh’ window, but they don’t have as many adjustments to make, significantly limiting total exposure to the electron beam before images are taken.
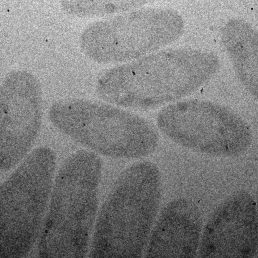
Next, the researchers proved that their device could prevent alteration of samples caused by overexposure to electron radiation. They imaged a bacterium called Cupriavidus metallidurans, a small single-celled organism that produces solid gold nanoparticles from aqueous gold tetrachloride, a potent heavy metal toxin to most organisms.
First, they imaged the bacteria by exposing it to increasing levels of radiation over the course of focusing and adjusting their settings before taking pictures. Then, they imaged a second batch of bacteria using their novel 25-window device. The images they produced showed significant differences.
“The images of cells exposed to higher levels of radiation were clearly different from cells imaged with no previous radiation exposure,” said James Evans, a senior scientist at Pacific Northwest National Laboratory and a co-author on the paper. “This proves that damage caused by being in the electron beam too long can cause artifacts that can yield false information. We saw much more pristine, undamaged cells using our multi-chamber device.”
Shokuhfar, a corresponding author on the paper, said the new device will also enable higher-fidelity imaging of nanoparticles using transmission electron microscopy. “Nanoparticles are also susceptible to damage from radiation, so this device will let us observe more accurately, how nanoparticles grow and change under different conditions, which has application in areas of new materials, nanoparticle interactions and medicine,” she said.
Hardeep Mehta and Ryan Kelly, from the Environmental Molecular Sciences Laboratory; and Chiwoo Park of Florida State University, are also co-authors on the paper.
This work was supported by the Department of Energy’s Office of Biological and Environmental Research Molecules to Mesoscale Bioimaging (project no. 66382) and was performed using Environmental Molecular Sciences Laboratory. Shokuhfar was supported by the National Science Foundation (CAREER Award DMR-1564950).